Cell-ebrity Drama: FoxN2, Hedgehog Signaling, and the Primary Cilia in Pancreatic Cancer
Cancer is a major worldwide health issue that causes immense suffering and loss of life. Out of different types of cancer, pancreatic cancer stands out, with its five-year survival rate of only 11.5% (Cancer of the Pancreas). Despite every effort, the development of a therapeutic treatment has been thwarted by pancreatic cancer’s aggressive nature, the rapid spread of tumor cells, and the absence of reliable diagnostic tools. The poor prognosis is linked to a lack of specific symptoms at the early stage and, therefore, progression to a later stage before proper diagnosis. In the rare instances when pancreatic cancer is detected early, it occurs during regular checkups or screening for unrelated issues. Much research into the biology of pancreatic cancer is in progress. One of the curious findings is a significant loss of primary cilia organelles associated with a progression to late-stage pancreatic cancer (Quilichini et al. 2019)
Within the cellular biology field, we encounter primary cilia, which are singular, antenna-like immobile organelles found in most cell types. Primary cilia play a pivotal role in various cellular functions, owing to their unique capacity to receive and respond to a wide array of external cellular signals. One example of such function happens in the context of Hedgehog (Hh) signaling during embryonic development. The Hh signaling pathway controls the growth and patterning of tissues and organs during development. When specialized signaling molecules known as Hh proteins attach to receptors on the cell membrane, they trigger a cascade of molecular events within the cilium. As a result, a signaling system that controls cell fate and tissue patterning during embryogenesis is activated. The capacity of primary cilia to transduce these signals is critical for appropriate limb creation, neural tube development, and other aspects of embryonic development, emphasizing their importance in orchestrating complex biological processes.
Recent studies have shown a link between primary cilia and each cancer hallmark (Liu, Kiseleva, and Golemis 2018; Fabbri and Mazure 2019). Such a connection is aiding scientists in clearly defining the role of cilia in cancer. For example, research has identified that inhibition of ciliogenesis, or the assembly and disassembly of cilia, is crucial for activating Hedgehog signaling and the progression of breast cancer to a more aggressive, metastatic disease (Hassounah et al. 2017). Research in Kristen Johnson’s lab at the University of New Hampshire at Manchester focuses on how a certain protein called FOXN2 may influence ciliogenesis and possibly impact the progression of pancreatic cancer to a more aggressive stage. My individual project, funded by a Summer Undergraduate Research Fellowship (SURF) from the Hamel Center for Undergraduate Research, explored how FOXN2 affects cilia number and structure and the role of Hedgehog signaling in a frequently studied mammalian cell line.
What kind of protein is FOXN2 and why do we care about it?
The process of ciliogenesis is intricately intertwined with the cell cycle. A fundamental aspect of the cell cycle is the regulation of the duplication of centrioles, small structures within the cell directing its division, and the accurate segregation of chromosomes during cell duplication. Centrioles are also crucial in cilia assembly. In the G0 phase of the cell cycle, a phase occurring outside of cell division, the mother centriole assumes a pivotal role by differentiating into a basal body and migrating to the cell’s plasma membrane, where it initiates cilia assembly. Notably, proteins that govern the centrioles’ duplication possess the capacity to inhibit the initiation of new ciliary assembly. Mutual regulation between cell cycle and ciliogenesis exists because of their correlation to centrioles. Considering cilia have multiple roles in development and physiology as well as intricacies of ciliogenesis and cell cycle processes and mechanisms, it is only plausible that defects in cilia would induce a multitude of diseases. Cilia defects have already been linked to several human disorders, including but not limited to polycystic kidney disease, retinal degeneration, and cancer (Duldulao, Li, and Sun, 2010).
Genes needed for normal cilia function can be influenced by transcription factors, which are proteins able to control which genes are “on” or “off.” The FOXN2 protein is a member of the forkhead family of transcription factors. These transcription factors play important roles in mediating expression of genes during organ development, cell metabolism, and immunoregulation, and, notably, in tumor formation and metastasis when their regulation falters (Bach et al., 2018; Golson and Kaestner,2 016). The dysregulation of FOXN2 has been linked to several cancers (Expression of FOXN2 in renal cancer, Ma et al. 2018, Nagel et al.2018); however, the detailed molecular means by which FOXN2 exerts its impacts on tumor formation still need to be researched. Recent computational analyses conducted by the Johnson laboratory, using RNA-Seq, have uncovered an intriguing novel connection between FOXN2 and genes associated with cilia. According to this data, we hypothesized that FOXN2 will serve as a negative regulator of ciliogenesis and possibly Hedgehog signaling. That means we predicted that expression of the FoxN2 gene in cells will result in fewer and shorter cilia. This in turn will negatively impact Hedgehog signaling and lead to dysregulation within the organism, possibly promoting cancer progression. (Note: For clarification, FOXN2 is a protein, and FoxN2 is a gene that codes for that protein. When we talk about expression, we talk about a gene, and when we talk about signaling or a regulator, we talk about a protein.)
The results derived from this research will be used to further advance our understanding of ciliogenesis and establish a clear connection between the expression of FOXN2 and the phenomena of cilia loss, as well as the possible association of FOXN2 with the Hedgehog signaling pathway. While mouse fibroblast cells were used as a model, these findings could be applied to human pancreatic cancer cells in the future, which could lead to the future development of alternative therapies for cancer treatment.
Methods and Preliminary Results
One of our research objectives included introducing FoxN2 to the mouse fibroblast cell line NIH3T3 and establishing a link between FOXN2 and ciliogenesis. This cell line is a prevalent model system for studying primary cilia. NIH3T3 cells are easy to maintain in culture and are extremely useful for molecular and cell biology research. Additionally, recent studies confirmed that NIH3T3 is one of the two most suitable cell lines to use while studying the Hedgehog signaling pathway (Gómez et al. 2022).
In cells, low serum concentration, or serum starvation, results in cell cycle arrest, which then leads to formation of primary cilia. Therefore, for the NIH3T3 cells to form primary cilia, we induced cell cycle arrest by culturing cells in a medium with a low serum concentration. According to previous studies, we expected ciliated NIH3T3 cells to peak around forty-eight hours after starvation. This was indeed shown, with 66% of our cells growing primary cilia after forty-eight hours.
Based upon our hypothesis of FOXN2 being a negative regulator of ciliogenesis, it was expected that overexpression (increased presence) of FoxN2 would lead to decreased numbers of cilia, centrioles, and cilia length in cells that express primary cilia, such as NIH3T3 cells. Lipofectamine transfection technique was used to overexpress FoxN2. This technique uses a lipid complex to encapsulate genetic material such as FoxN2 and pass through protective layers, delivering it into the cell. To visualize the results, I stained cilia using the immunofluorescence staining technique and observed change in cilia under a fluorescent microscope. The preliminary data obtained through immunofluorescence staining offers compelling insights into the regulatory role of FOXN2 in ciliogenesis. NIH3T3 cells that overexpressed FoxN2 showed a discernible decline in the number of cilia present. This evidence corroborates the working hypothesis, indicating that FOXN2 serves as a negative regulator of ciliogenesis.
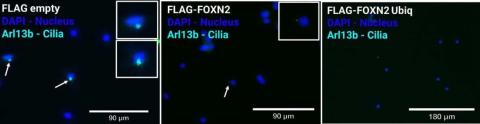
Immunoflurescent detection of primary cilia in NIH3T3 cell line with transfected FLAG-empty (control), FLAG-FOXN2, FLAG-FOXN2 Ubiquitnation mutant plasmids following 48-hour serum starvation. Cilia was stained with Arl13b (mouse) primary Ab and AF 647 (anti-mouse) secondary Ab. AF 647 is false colored with cyan. Cells transfected with FLAG-FOXN2 shows ciliation compared to FLAG empty, and FLAG-FOXN2 Ubiq has no cilia detected.
As part of our aim, I also explored a ubiquitin-deficient mutant of FoxN2. Degradation of FOXN2 has previously been studied in the context of lung cancer, and it was found that FOXN2 is broken down with the help of the regulatory protein ubiquitin, which tags proteins to be degraded. To further investigate this finding, scientists generated a FoxN2 ubiquitin-deficient mutant that made the FOXN2 protein more stable (Ma et al. 2018). Even though this mutant has not been studied in the context of ciliogenesis and pancreatic cancer, we expected the presence of this mutant FOXN2 in the cells would allow for an increased negative effect on ciliogenesis. To create a mutant version of FoxN2, I used molecular cloning techniques to generate plasmids (small circular pieces of DNA) containing a change in the DNA that coded for a version of FoxN2 that could not be degraded. I conducted analogous experiments involving its overexpression within NIH3T3 cells. We observed that cells containing this mutant exhibited a perceptible reduction in cilia number when compared with cells with the regular form of FOXN2. This provides further substantiation that when FOXN2 is stable, it exerts a more pronounced inhibitory influence on ciliogenesis. This further supports our hypothesis that FOXN2 negatively regulates ciliogenesis, and by doing so, its presence could possibly speed up the progression of pancreatic cancer. Theoretically, for cancer treatment, we could develop something that increases ciliogenesis, for example by reducing the levels of FOXN2.
Another research objective was to establish the connection between FOXN2 and Hedgehog signaling. To do this, we added a Smoothened Agonist to FOXN2 expressing NIH3T3 cells. When Smoothened Agonist binds to the Smoothened protein, it can turn on Hedgehog signaling, mimicking the natural signals sent by cilia. It is imperative to note that experiments assessing the response of cells with activated Hedgehog pathways have yielded inconclusive results so far. Further investigations are warranted to elucidate this aspect comprehensively.
What Is Next?
Although our complete findings are forthcoming, further research on the novel connection between FOXN2 and ciliogenesis is warranted. Going forward, the Johnson lab plans to replicate immunofluorescence staining experiments and study protein expression, protein-protein interactions, and activation of signaling pathways using Western blotting experiments. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR), a molecular biology technique that quantitates the amounts of certain genes present in the cell, will be used to determine cells’ response to Hh pathway stimulation.
Additionally, experiments with different FOXN2 mutants will be studied. While ubiquitin-deficient FoxN2 mutant results in stabilized expression of FoxN2, a DNA binding domain FoxN2 mutant is hypothesized to do the opposite. The mutation in the DNA binding domain prevents the FOXN2 binding of DNA, a requirement for its job as a transcription factor. With lack of functioning FOXN2, we might expect to see normal ciliogenesis. While ubiquitin-deficient FoxN2 mutant serves only as a tool to support our hypothesis, DNA binding domain FoxN2 mutant additionally has implications to be developed into a treatment. Conceptually, if normal FoxN2 is replaced by DNA binding domain FoxN2 mutant in the pancreatic cells, it will induce normal ciliogenesis and slow the progression of the disease, which could aid in developing alternative therapies for pancreatic cancer treatment.
The experience of proposing my own research project, attaining results to find an answer to an unanswered question, and presenting it to the greater scientific community solidified my aspirations to pursue research as a career. The ever-evolving nature of research is an intellectually rewarding journey with endless opportunities, and pushing the boundaries of human knowledge sounds like a rewarding endeavor. I have recently interviewed for biomedical sciences Ph.D. programs, and I am considering a couple of offers. Research offers dynamic, interdisciplinary, and innovative opportunities for those passionate about knowledge. I want to be a part of shaping the future, leaving a lasting impact on our understanding of the world and biology in particular.
I would like to thank my faculty mentor, Dr. Kristen Johnson, for her unwavering guidance and support throughout my journey at UNH. Her patience, encouragement, and insight were invaluable in this research project. I would also like to extend my gratitude to Dr. Johnson’s lab members, with whom I was delighted to share this fantastic experience. Thank you to the Hamel Center for Undergraduate Research staff and Mr. Dana Hamel for their financial support through a Summer Undergraduate Research Fellowship (SURF).
References
Bach, D.-H.; Long, N. P.; Luu, T.-T.-T.; Anh, N. H.; Kwon, S. W.; Lee, S. K. The Dominant Role of Forkhead Box Proteins in Cancer. Int J Mol Sci 2018, 19 (10), 3279. https://doi.org/10.3390/ijms19103279.
Cancer of the Pancreas - Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/pancreas.html
Duldulao, N. A., Li, J., & Sun, Z. (2010). Cilia in cell signaling and human disorders. Protein & Cell, 1(8), 726–736. https://doi.org/10.1007/s13238-010-0098-7
Expression of FOXN2 in renal cancer - The Human Protein Atlas. https://www.proteinatlas.org/ENSG00000170802-FOXN2/pathology/renal+cancer.
Fabbri, L.; Bost, F.; Mazure, N. M. Primary Cilium in Cancer Hallmarks. Int J Mol Sci 2019, 20 (6), 1336. https://doi.org/10.3390/ijms20061336.
Golson, M. L.; Kaestner, K. H. Fox Transcription Factors: From Development to Disease. Development 2016, 143 (24), 4558–4570. https://doi.org/10.1242/dev.112672.
Gómez, A. E., Christman, A. K., Van De Weghe, J. C., Finn, M., & Doherty, D. (2022). Systematic analysis of cilia characteristics and Hedgehog signaling in five immortal cell lines. PLOS One, 17(12), e0266433. https://doi.org/10.1371/journal.pone.0266433
Hassounah, N. B.; Nunez, M.; Fordyce, C.; Roe, D.; Nagle, R.; Bunch, T.; McDermott, K. M. Inhibition of Ciliogenesis Promotes Hedgehog Signaling, Tumorigenesis, and Metastasis in Breast Cancer. Mol Cancer Res 2017, 15 (10), 1421–1430. https://doi.org/10.1158/1541-7786.MCR-17-0034.
Liu, H., Kiseleva, A. A., & Golemis, E. A. (2018). Ciliary signaling in cancer. Nature Reviews: Cancer, 18(8), 511–524. https://doi.org/10.1038/s41568-018-0023-6
Ma, J.; Lu, Y.; Zhang, S.; Li, Y.; Huang, J.; Yin, Z.; Ren, J.; Huang, K.; Liu, L.; Yang, K.; Wu, G.; Xu, S. β-Trcp Ubiquitin Ligase and RSK2 Kinase-Mediated Degradation of FOXN2 Promotes Tumorigenesis and Radioresistance in Lung Cancer. Cell Death Differ. 2018, 25 (8), 1473–1485. https://doi.org/10.1038/s41418-017-0055-6.
Nagel, S.; Pommerenke, C.; Meyer, C.; Kaufmann, M.; MacLeod, R. A. F.; Drexler, H. G. Identification of a Tumor Suppressor Network in T-Cell Leukemia. Leuk. Lymphoma 2017, 58 (9), 2196–2207. https://doi.org/10.1080/10428194.2017.1283029.
Quilichini, E.; Fabre, M.; Dirami, T.; Stedman, A.; De Vas, M.; Ozguc, O.; Pasek, R. C.; Cereghini, S.; Morillon, L.; Guerra, C.; Couvelard, A.; Gannon, M.; Haumaitre, C. Pancreatic Ductal Deletion of Hnf1b Disrupts Exocrine Homeostasis, Leads to Pancreatitis, and Facilitates Tumorigenesis. Cellular and Molecular Gastroenterology and Hepatology 2019, 8 (3), 487–511. https://doi.org/10.1016/j.jcmgh.2019.06.005.
Author and Mentor Bios
Nadine Emil’, from Manchester, New Hampshire, is a member of the Millyard Scholars program and the TriBeta National Biological Honors Society. She graduated from the University of New Hampshire at Manchester in December 2023 with a bachelor of science in biotechnology. She did her research with a Summer Undergraduate Research Fellowship provided by the Hamel Center for Undergraduate Research and continued this project into the fall semester, presenting her results as a part of her senior capstone project. Nadine joined Dr. Johnson’s lab because she admired her teaching and guidance, and wanted to learn more from her. The research topic of pancreatic cancer, which is known for being aggressive, furthered Nadine’s interest. She was also curious to develop her own research proposal and pursue a hypothesis of interest. Nadine learned a lot to further understand what experiments to conduct, their purpose, and how to troubleshoot. She explored topics such as molecular mechanisms, signaling pathways, and their role in human disease, and she hopes to study them more in the future. Although the research was difficult at times, Nadine found the troubleshooting process to be fun and enjoyed the challenge. She submitted her research to Inquiry as a way to challenge herself to effectively present results to a general audience. She wants to continue pursuing research throughout her career and is planning to enter a Ph.D. program in the fall. She is happy to have gained the valuable experience of conducting independent research, presenting her results, and publishing her findings.
Dr. Kristen C. Johnson is an associate professor in the Department of Life Sciences at the University of New Hampshire at Manchester, where she has been teaching since 2017. Her research specialization is in cancer biology, and she teaches cell and molecular biology and biotechnology. Dr. Johnson studied cancer as a graduate student and has always been passionate about research in this area. Her current research project is a result of a collaboration with a friend from Columbia University. She has mentored over fifty undergraduate researchers, with Nadine being her first Inquiry author. Dr. Johnson describes this project as having its successes and failures, like any other, yet Nadine was able to roll with the ups and downs and adapt accordingly. Dr. Johnson has been fortunate to work with many great undergraduate research students and is proud that each has taken their role seriously and worked hard in the lab. “Nadine is a very talented young woman who is passionate about research,” said Dr. Johnson. “I am excited for Nadine’s next adventures as she embarks on graduate school in the fall!”
Copyright 2024 © Nadine Emil’
