Mane Pulling and Stress Response: Investigating the Welfare Impact of an Equine Husbandry Practice

The author, Rowan Grzeszkiewicz-O’Neill
As someone who works with animals, I feel a large responsibility to look after their needs and ensure their comfort. This responsibility encompasses many things, including continually educating myself and others on the best practices for animal welfare. This sometimes requires scrutinizing practices that possibly cause harm, as indicated either by research or by personal experience. In fall 2024, I had the opportunity to do my own investigation into one such practice, and I took it.
The practice I researched is mane pulling. This is a technique used to make horses “show ready” for English riding such as dressage or show jumping by thinning their manes so that they look even and can be more easily braided. The traditional method of mane pulling involves teasing out groups of hair to wrap around a small metal comb, then pulling down on the comb to rip the hair out at the root. This is done in small sections all the way down the horse’s mane, until the desired length and thickness of hair is achieved. Naturally, this practice is controversial. It involves what we know as a painful sensation and is done only for aesthetic purposes.
Many of the equestrians familiar with mane pulling avoid using this technique or condemn it altogether, because to them it seems obvious that having hair ripped out at the root hurts a horse. However, some argue that this is anthropomorphism, the attribution of human characteristics to nonhumans. How can we know that horses experience the sensation of having hair pulled out the same as we do? After all, pain is not always triggered and experienced the same way in other species, and there has been no published research on the practice of mane pulling. There are also many horses that will stand still to have their manes pulled.
As an equine science student and equestrian who has observed this practice multiple times and felt it looked painful, I was interested to learn how mane pulling actually impacts horses. However, an online search on “mane pulling” brought up no published results, only blogs and other webpages with contrasting opinions. I decided to investigate this research question myself, with guidance from Sarah Rigg, former director of the Equine Program at the University of New Hampshire (UNH). My goal was to assess how horses respond to mane pulling and to evaluate whether these responses are indicative of pain.
Pain Perception and Detection
The research on equine nociception and pain is extremely limited. Nociception is the process by which the nervous system detects damaging stimuli and turns it into a signal that reaches the nervous system, where it is then processed as pain in animals with the brain structures to do so. For example, in mammals the neocortex part of the brain is associated with pain. In birds, which don’t have a neocortex, pain is felt because of the similar structure of neural fibers in their pallium. Some researchers believe that fish lack any conscious feeling of pain because of the relative simplicity of sensory processing structures in their brain and instead respond to noxious stimuli only as an innate reflex triggered by nociception (Key 2014), although this is a contentious subject. It is difficult to parse out these distinctions, because nociception varies by species, and pain is a subjective experience that varies by individual, making it highly difficult to study.
Subjective perception of pain in horses has not been studied as of this writing, and only within recent years have the nociceptive structures of equine skin been researched. In 2020, it was found that the epidermis, the outer layer of skin that detects pain, has the same thickness and nociceptive nerve concentration in horses as in humans (Tong et al.), indicating that a horse’s skin sensitivity to nociception is similar to ours. However, this does not tell us how a horse experiences the signals detected by nociceptors or give us insight into the effects of specific stimuli.
For the time being, the only way to gauge whether a particular stimulus is causing pain in a horse is to analyze physiological and behavioral indicators. I sought to do this in my research by using heart rate, behavioral indicators, and eye temperature to assess how horses respond to mane pulling. These parameters are all commonplace in equine welfare research, because they reliably indicate stress. In mammals, acute pain excites the sympathetic nervous system, a network of nerves that trigger a series of physiological changes and create the “fight or flight” response (Burton et al. 2016). One physiological change that occurs in horses is a rapid increase in core body temperature, which is influenced by alterations to blood flow; this change is mirrored in certain locations such as the eyes and can be measured by infrared thermography, which is becoming an increasingly prevalent method of assessing stress response in horses (Yarnell et al. 2013). Heart rate also quickly increases with sympathetic nervous system excitation and is commonly used as an indicator of stress when assessing equine welfare (Börstel et al. 2017).
Specific sets of behavior known to be linked to pain or stress can also be used as an indicator. Behavior can be quantitatively assessed with an ethogram, a list of species-specific actions. Ethograms can contain very general behaviors, such as walking and sleeping, or can be limited to actions that hint at an animal’s psychological state, such as a tilted head or repeatedly swishing tail being used to judge possible pain in a ridden horse.
I used these three parameters to experimentally determine how horses respond to mane pulling and theorize about how they experience the practice. I hypothesized that horses would experience pain from mane pulling, which would activate their sympathetic nervous systems and result in increases to their heart rate, frequency of displaying stress behaviors, and eye temperature.
Methods
I used seven horses of different breeds ranging in age from fourteen to twenty-three, all of which were a part of the UNH Equine Program and housed at the UNH equine facilities. Five horses were male and two were female. They were all accustomed to routine handling, such as basic restraint and grooming, because of regular use in equestrian activities by UNH students, including myself. Use of horses in this study aligned with the guidelines and protocols established for equestrian activities at UNH by the Institutional Animal Care and Use Committee (IACUC).
I conducted tests over three consecutive weekends, during which horses are kept in turn-outs throughout the day and not used for equestrian activities. Turn-outs are outdoor areas enclosed by fencing that allow space for the horse to run. I tested each horse at approximately the same time each weekend, to control for the influence of factors such as daily routine and fluctuations in cortisol levels caused by the effect of circadian rhythm. During testing, I brought horses into the barn and placed them on cross-ties, a restraint system in which two ropes or straps are clipped to either side of a halter placed on the horse’s head. There is slack in the cross-ties to allow the horse freedom of motion of the head and the space to take a few steps in any direction.
Horses were tested under three conditions, one of which was experimental and two of which were controls. In the experimental condition, horses had their manes pulled by various members of the UNH Intercollegiate Horse Show Association (IHSA) team using the traditional method described. During the first control condition, which I will refer to as the “baseline condition,” I moved the horses into the barn and placed them on cross-ties but they were not handled further. During the second control condition, members of the IHSA brushed but did not pull the horses’ manes. I collected data for eleven minutes under each of the three testing conditions, with the exception of one horse who began to exhibit stress behaviors that posed a safety risk and caused the IHSA handler to stop pulling his mane at six minutes. I collected heart rate, eye temperature, and behavioral data during each test over the three consecutive weekends. Each horse was tested under all three conditions with no replications of the treatment.
Heart Rate
I measured heart rate with a Polar H10 Equine Heart Rate Sensor. First, I used a sponge to apply water to the left side of the horse’s chest and rubbed electrode gel into the horse’s fur to enhance conductivity. I then placed the sensor in this area, just behind the elbow, and used a chest strap to hold it in place. The sensor connected to my phone and displayed the horse’s heart rate in real time. I recorded this value every twenty seconds over a span of ten minutes, starting when mane pulling or brushing began in the conditions involving handling, and after the horse had been secured in the cross-ties in the baseline condition. Horses did not appear to react to the sensor beyond sometimes turning their head to watch as I placed the sensor before testing.
Behavior
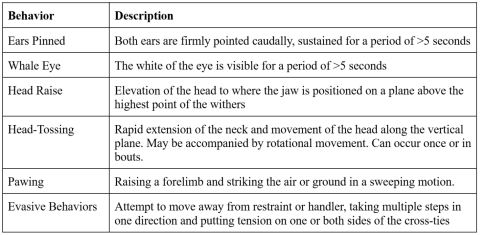
Figure 1: The stress behavior ethogram used in data collection.
To evaluate behavior, I used a camcorder to film each data collection session for later analysis. I created an ethogram (Figure 1) containing pain- and stress-related equine behaviors based on literature research (see Young et al. 2012; Hall & Heleski 2017; Torcivia & McDonnell 2021). I listed six readily identifiable behaviors in the ethogram: ear pinning, whale eye, head raising, head tossing, pawing, and evasive behaviors. Ear pinning involves flattening the ears back against the head, whale eye is when the whites of the horse’s eye are shown, and pawing involves the horse striking the air or ground with a hoof. Head tossing is distinguished by raising the head in a repetitive and quick movement. The ethogram provides detailed definitions for all terms, which allowed me to accurately identify stress behaviors by a specific set of criteria.
I watched the videos and recorded each time a behavior occurred. I did not record data at the points when I was in the stall using the thermal camera to assess eye temperature, because some horses would raise their heads while it was being used, which would have been recorded as a sign of stress. I also excluded points where the horse moved out of full view of the camera. Because of this, I ran a stopwatch while recording data to find the length of footage that I actually used. I summed up the total number of stress behaviors displayed, then divided this by the length of footage in minutes to find the rate of stress behaviors exhibited per minute of testing.
Eye Temperature
A FLIR C5 thermal camera was used to collect temperature data using infrared thermography. I took thermal photos of the left side of the horse’s head at minute one, minute six, and minute eleven of data collection. Images were taken of the left side of the horse’s face, at approximately a 90-degree angle and 60 centimeters away, and focused on the horse’s eye. I took several photos at each time point to ensure that there would be at least one usable image. I often had to stand still and wait for the horse to acclimate to the camera, because some horses were curious about the device and would turn their heads to examine it.

Figure 2: One of the infrared thermographic images used in the study.
I analyzed the images using FLIR Ignite software. First, I judged the images based on clarity and how close they were to the angle and distance I intended. I selected the highest-quality image from each time point in order to have three images per test. I then made adjustments to each of the three images. Each image was calibrated to an emissivity of 0.98, which is the emissivity of mammalian skin. Emissivity is a measure of an object’s ability to emit heat and affects how thermal imaging “reads” the temperature of the object. I also calibrated the ambient temperature and humidity on the images using data I had recorded from the Unni UN0581 thermometer and hygrometer in the barn.
I then used the ellipse measurement tool in the FLIR Ignite editor to select and find the average temperature at the lacrimal caruncle, the structure in the inner corner of the eye (Figure 2). I measured temperature at this area because the lacrimal caruncle is generally considered the optimal location to measure eye temperature; its rich vascularization with capillaries controlled by sympathetic innervation results in rapid changes to temperature in response to sympathetic nervous system activation (Kim & Cho, 2021). I averaged the temperatures of the three images to find one temperature value for each test.
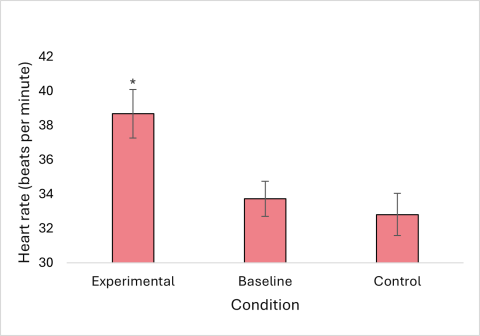
Figure 3: Average heart rate of horses during mane pulling, while unhandled, and during mane brushing. Error bars represent a 95% confidence interval. Asterisks represent a statistically significant difference between the marked condition and both other conditions.
Data Analysis and Results
After data collection, I used a one-way ANOVA test for each parameter to determine if there were significant differences between the conditions. Then, a Tukey-Kramer post hoc test was used to find which conditions differed significantly, because an ANOVA test determines only whether there is a significant difference and not where this difference lies. I performed all data analysis in Microsoft Excel and used the Analysis ToolPak add-in for the ANOVA tests. I excluded the eye temperature data from one horse before data analysis, because during the study she developed an eye condition that caused inflammation.
While having their manes pulled, all horses exhibited an increase in average heart rate and number of stress behaviors per minute, and the highest average eye temperature for the group was seen under this condition. The difference between the heart rate during the experimental condition and other conditions was statistically significant (Figure 3). The increase in the rate of stress behaviors exhibited during the experimental condition was also statistically significant (Figure 4). There was not a significant difference between eye temperatures in any of the conditions (Figure 5).
Discussion
The results indicate that mane pulling is likely painful. The heart rate data and behavioral data both showed that mane pulling was an adverse experience for the horses tested. Each horse had an increased heart rate and exhibited stress behaviors more frequently while having their mane pulled, which indicates that they all experienced stress as a result of the sensation it caused.

Figure 4: Average number of stress behaviors per minute, exhibited by horses experiencing mane pulling (experimental condition), no handling (baseline), and mane brushing (control). Error bars represent a 95% confidence interval. Asterisks represent a statistically significant difference between the marked condition and both other conditions.
The level of pain or other discomfort caused by mane pulling may vary by individual. The amount by which average heart rate and rate of stress behaviors changed during mane pulling was highly variable by horse, which may mean that some horses are more uncomfortable during the process than others. For instance, the horse that had his mane pulled for only six minutes exhibited the most stress behaviors of all the horses during the experimental condition and was persistent in trying to avoid his handler by moving away and knocking his body against her. He was much calmer during the two control conditions, and his heart rate was fifteen beats per minute lower while he was standing by himself in the stall and seventeen beats per minute lower when his mane was being brushed. Another two horses that I was hoping to include in the study had to be excluded after they immediately exhibited such extreme stress behaviors in response to mane pulling that their handlers decided not to proceed. In contrast, one horse studied had an increase of only three beats per minute and exhibited mostly undisruptive stress behaviors such as ear pinning during mane pulling.
It is also possible that the difference in reactions is partially caused by different temperaments; horses may have had similar sensations but experienced different levels of mental distress, or the sympathetic nervous systems in some horses may be more strongly triggered by the same level of stimulation. It is also possible that some of the horses used had previously had their manes pulled, which may affect their sensitivity to the process. Unfortunately, it is not possible to understand the nuances of the individual experiences of horses from the given information. Regardless, all horses did show changes to heart rate and stress behaviors. This indicates that having hair pulled out is to some degree an uncomfortable or painful experience for horses.
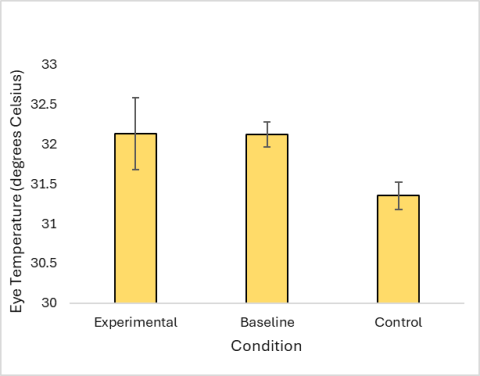
Figure 5: Average eye temperature of horses during mane pulling, while unhandled, and during mane brushing. Error bars represent a 95% confidence interval.
There are limitations to how these findings can be applied, which arise from both the methods and the results. The sample size was small, and the group was of mixed breed, age, and sex; these horses are likely not a good representation of the entire population. In addition, I conducted this research at the UNH equine facilities, a location that does not provide a controlled environment. The barn is often busy with visitors walking down the aisle and the noise of facility machinery in the background, which may create outside sources of stress.
There were also multiple technical challenges associated with the use of infrared thermography in this project. It took time for me to adjust to using the thermal camera on horses. I noticed that the images I took later in the study, when I had had more practice with the camera, were of higher quality. Additionally, there was a major flaw in my methodology: The ambient temperature differed drastically between weekends, ranging from 54 to 75 degrees Fahrenheit. While this does not have much impact on core body temperature in mammals because of strict thermoregulation, environmental temperature does impact body temperature at the surface level, which impacts the use of infrared thermography (Taylor et al. 2014).
Despite its flaws, many insights can be drawn from this study. These results call into question the ethics of a practice that is relatively common in horse showing. Mane pulling is used solely for aesthetic reasons, and these findings show that it has a negative impact on welfare. Many people willing to pull a horse’s mane believe that it causes no pain or a negligible amount of discomfort, but for the first time, there is now empirical evidence of how mane pulling impacts horses and this evidence indicates that it is painful. Most of the horses studied were willing to stand still and tolerate mane pulling, with some exhibiting only subtle external signs of stress; 38 percent of recorded behaviors were pinned ears and whale eye, which can be easily missed. Nevertheless, the heart rate data suggests that all were experiencing some level of stress. These findings are difficult to apply to all horses, but they should bring people pause: Is it ethical to take actions that have been shown to cause pain to animals to alter an animal’s appearance for the sake of competition that exists only for human entertainment?
This is just one potentially harmful practice that is used for human gain and founded on assumptions about what a given animal does and does not experience. It is far from being an isolated example. Other practices that may compromise equine welfare, such as lack of pasture access for many horse herds and highly frequent use of physical punishment in some training methodologies, are prevalent in sport and in husbandry.
Furthermore, concerns about animal welfare are certainly not limited to the equine industry. Humans have fundamentally altered the lives of animals by holding them in captivity and domesticating them for companionship, food, research, and entertainment. As such, we have a moral responsibility to ensure their well-being. It is imperative that this responsibility be upheld by seeking to understand the needs and experiences of animals and using this knowledge to reduce welfare-compromising practices.
Reflection
On the first day of the fall semester, in my Animal Science 795 Investigations course, I learned that I would be provided with an equine heart rate monitor for an assigned group project on equine breathing. Knowing that I would have access to this instrument, and that the horses at the UNH equine facility would soon have their manes pulled for upcoming equestrian events, I excitedly approached the instructor, Sarah Rigg, with my idea for independent research on stress response to mane pulling. My project came together with remarkable speed, and in less than two weeks I was at the barn with my makeshift assemblage of scientific equipment, excited to begin my own research.
There were many trials and tribulations that accompanied the scientific process. I found flaws in my experimental design, my sample size shrank, and the hot early September weather was draining. But seeing the project come together brought me a sense of satisfaction like no other, especially after so much time wanting to be involved in research but not knowing where to start. I am very glad that I created this learning experience for myself and that I took the opportunity to conduct independent research. I intend to do more undergraduate research for my Honors thesis, which will be a larger project, and I now feel better prepared for doing so. I also plan to continue my involvement in research past my bachelor’s degree and will soon be applying to dual DVM/PhD programs to pursue my interests in both research and veterinary medicine. This experience helped me develop my experimental design skills and feel more confident in my decision to apply to graduate school.
While this was an independent project, I could not have done it alone. I would like to thank Sarah Rigg, both for mentoring this project and for her continued support, guidance, and encouragement that push me to pursue my academic interests and accomplish things that I previously never thought possible. I would also like to thank Dr. Jarema for her guidance on statistical testing, and Dr. Curren for her advice on ethogram creation and for her enthusiasm and interest that helped fuel my passion for this project. I also must express my gratitude for my IHSA teammates, the horse handlers: Without them, this project would not have been possible.
References
Börstel, U. K., Visser, E. K., & Hall, C. (2017). Indicators of stress in equitation. Applied Animal Behaviour Science, 190, 43–56. https://doi.org/10.1016/j.applanim.2017.02.018
Burton, A. R., Fazalbhoy, A., & Macefield, V. G. (2016). Sympathetic responses to noxious stimulation of muscle and skin. Frontiers in Neurology, 7. https://doi.org/10.3389/fneur.2016.00109
Hall, C. & Heleski, C. (2017). The role of the ethogram in equitation science. Applied Animal Behaviour Science, 190, 102–110. https://doi.org/10.1016/j.applanim.2017.02.013
Key, B. (2014). Fish do not feel pain and its implications for understanding phenomenal consciousness. Biology & Philosophy, 30, 149–165. https://doi.org/10.1007/s10539-014-9469-4
Kim, S. M. & Cho, G. J. (2021). Validation of Eye Temperature Assessed Using Infrared Thermography as an Indicator of Welfare in Horses. Applied Sciences, 11(16). http://dx.doi.org/10.3390/app11167186
Taylor, N. A. S., Tipton, M. J., & Kenny, G. P. (2014). Considerations for the measurement of core, skin and mean body temperatures. Journal of Thermal Biology, 46, 72-101. https://doi.org/10.1016/j.jtherbio.2014.10.006
Tong, L., Stewart, M., Johnson, I., Appleyard, R., Wilson, B., James, O., Johnson, C., & McGreevy, P. (2020). A comparative neuro-histological assessment of gluteal skin thickness and cutaneous nociceptor distribution in horses and humans. Animals, 10(11). https://doi.org/10.3390/ani10112094
Torvicia, C. & McDonnell, S. (2021). Equine discomfort ethogram. Animals, 11(2). https://doi.org/10.3390/ani11020580
Yarnell, K., Hall, C., & Billett, E. (2013). An assessment of the aversive nature of an animal management procedure (clipping) using behavioral and physiological measures. Physiology & Behavior, 118, 32–39. https://doi.org/10.1016/j.physbeh.2013.05.013
Young, T., Creighton, E., Smith, T., & Hosie, C. (2012). A novel scale of behavioural indicators of stress for use with domestic horses. Applied Animal Behaviour Science, 140(1–2), 33–43. https://doi.org/10.1016/j.applanim.2012.05.008
Author and Mentor Bios
Originally from Los Altos, California, Rowan Grzeszkiewicz-O’Neill is an equine science major at the University of New Hampshire who will graduate in 2026. They are minoring in zoology and business administration. At UNH, Rowan is a member of the Hamel Honors and Scholars College, Phi Sigma, and the IHSA equestrian team.
Sarah Hamilton Rigg has taught at UNH since 2000 and served as director of the Equine Program from 2006 until 2022. She coached the UNH dressage team to a national championship in 2009 and is a dressage technical delegate for the United States Dressage Federation. Her areas of interest include horsemanship, the systematic development of horse and rider, pedagogical development of the riding instructor, and the horse in history, art and culture.
Copyright 2025 © Rowan Grzeszkiewicz-O’Neill