Playing with Sea Monkeys: An Examination of the Use of Decapsulated Artemia as a Means of Improving Current Zebrafish Culture Practices
Zebrafish (Danio rerio) is an ornamental species that has been used widely as a vertebrate model organism for research in the genetics, human health, environmental health, neurobiology, and toxicology fields (Bambino & Chu, 2017; Howe et al., 2013; Kalueff et al., 2014; Major & Poss, 2007; Modarresi Chahardehi et al., 2020). Its advantages of high fertility, short reproductive cycle, and fully sequenced genome have enabled breakthroughs in medical research in the areas of heart disease, cancer, and many others (Howe et al., 2013; Major & Poss, 2007). However, lab culturing of zebrafish poses many challenges, especially in its larval stage.

Due to their small size at hatching and poorly developed digestive tract, larval zebrafish require live food such as rotifers for the first two to five days of feeding (Conceição et al., 2010; Houde, 1972; Nusslein-Volhard & Dahm, 2002). Rotifers (Brachionus plicatilis) are planktonic organisms that require a separate culture that is both labor intensive and expensive (Houde, 1972; Ma et al., 2023). Rotifer care utilizes continuous culture, which requires daily care and daily growth of new generations produced by the older batches of maturing generations. It is reliant on a variety of factors such as food supply and removal of ammonia from the culture; this causes it to be unpredictable, which can lead to rotifer population collapses and a sudden loss of food supply for the fish it is being fed to (Conceição et al., 2010; Ma et al., 2023). This study, which was funded by an Undergraduate Research Award from the Hamel Center for Undergraduate Research, aimed to simplify the current larval zebrafish feeding regimen to promote the successful use of this model organism across a wide range of laboratories, including on the UNH campus. To fulfill this goal, Dr. Kwasek and I evaluated the effect of replacing rotifers with decapsulated Artemia nauplii (decaps) on the short-term and long-term growth and survival of larval zebrafish.
Decapsulated Artemia nauplii has a small width (like rotifers), which led us to believe that despite their larger length, decaps would be a suitable food for the small mouth size of larval zebrafish and would not jeopardize their growth and survival. The decapsulation process involves removing the hard outer layer of the cyst, called the chorion, which allows the nauplii to hatch earlier and at a smaller size (Sorgeloos et al., 1977). Decapsulated Artemia nauplii is also a form of live feed that must be cultured; however, it uses on-demand culture rather than continuous culture. On-demand culture consists of hatching cysts (eggs) within a 24-hour period in a controlled setting. In addition to being much easier to cultivate, the decapsulation process for Artemia nauplii disinfects cysts, providing an additional benefit because rotifers can be disease vectors (Sorgeloos et al., 1977). Once the larval zebrafish are large enough to consume larger live feeds, they are typically transitioned to regular, capsulated Artemia nauplii. Therefore, giving them decaps during the first two to five days of feeding would also provide them with the taste of Artemia, which we predicted would assist in the transition from the previous feed (rotifers) to the new (nauplii), reducing starvation-caused mortality (Conceição et al., 2010; J. Dhont et al., 2013).
Larval Zebrafish Study Execution
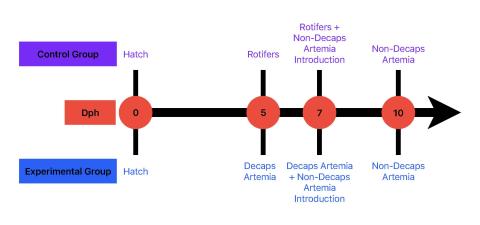
Figure 2. The feeding regimen used for both experimental and control groups from days 0-10.
Our study started with 1200 larval zebrafish randomly split into six 4.5L tanks, allowing three replicate tanks per group at a density of 200 larvae per tank. It was executed utilizing a highly controlled environment within a zebrafish culture system (Iwaki Aquatic, MA) using zebrafish larvae at swim-up stage, four days post-hatch (dph). There were two groups in the study: a control representing the current optimal feeding regimen of rotifers transitioned to capsulated Artemia nauplii (regular protocol) and an experimental group where rotifers were substituted with decapsulated Artemia nauplii (decaps protocol).
At 10 dph and at the end of the study (13 dph), I measured the body weight and body length of all fish. I also assessed survival (% mortality) through a daily log of observed mortalities. Each tank served as an observation unit during data collection, and I measured water quality in the tanks daily. The average temperature recorded was 27.8ºC and the average pH recorded was 8.30. I fed all fish to satiation and the study lasted until all fish metamorphosed into their juvenile stage (total duration of thirteen days). At the conclusion of data collection, I aggregated the data and completed T-test statistical analyses of independent samples to determine significance.
Preliminary Findings
We concluded that larval zebrafish who were fed decaps showed a significantly higher percent survival at both ten days post-hatch and thirteen days post-hatch. Fish in this group also showed a significantly higher weight at thirteen days post-hatch. There was no significant difference in body length between larval zebrafish experiencing the regular protocol and those experiencing the decaps protocol, however fish that were fed decaps on average tended to be longer. In all study parameters (body length, weight, and survival), fish in the decaps protocol performed equal to or better than fish in the regular protocol. (See Figure 4, below.) For that reason, we suggest that labs cultivating larval zebrafish have an option of transitioning to feeding with decapsulated Artemia nauplii in place of the traditionally used rotifers. Doing so should have significant implications across a variety of fields by simplifying and increasing the accessibility of culturing zebrafish for research requiring this model organism.
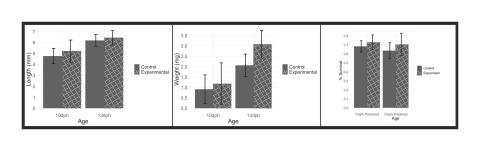
Figure 4 (click to enlarge). Error bars indicate standard deviation from the mean. From left to right: Length (mm) for both treatment groups at both sampling ages. Neither of the relationships between treatment groups were significant (10dph Tcalc = 1.78, 10dph df = 117; 13dph Tcalc = 1.03, 13dph df = 302). Weight (mg) for both treatment groups at both sampling ages. Only the 13dph relationship between treatment groups was significant (10dph Tcalc = 1.66, 10dph df = 114; 13dph Tcalc = 13.07, 13dph df = 302). Percent survival for both treatment groups at sampling ages. All relationships between control and experimental were significant (10dph Tcalc = 5.60, 10dph df = 337; 13dph Tcalc = 5.78, 13dph df = 337). Predicted refers to survival based on daily mortality counts.
Reflection
This study gave me a better understanding of what it means to care for a large number of aquatic organisms. Larval fish especially require a lot of time and care, from feeding five times a day, to siphoning debris from the bottom of the tank without siphoning the fish that are small enough to be siphoned and not aware that they should move away from the siphon. Caring for and cultivating live feeds on top of that gave me a full-time job and valuable time management skills as I fit in other tasks such as food prep and water chemistry tests between my five daily feeds.
I learned many things from this experience, from how to write a research proposal, to how to troubleshoot in short amounts of time without compromising the integrity of a study. I additionally learned about budgeting, animal husbandry, aquatic life support systems, and the scientific publication process. On a more personal level, I learned that I am especially interested in fish movement and behavior, something that is easier to study in larger or more developed fish, but just as necessary to study in the smaller, less developed larval stages. This will inform my future research directions as I explore more areas within the realm of marine biology. I also learned that I enjoy fish husbandry, working with zebrafish, and aquaculture, so I would consider doing another study within this area of marine biology, likely with adult zebrafish or another species of fish. This study gave me ideas and questions to answer in future studies, and I look forward to answering some of them using the skills I have learned.
I would like to extend my gratitude first to the Kwasek Lab (Dr. Karolina Kwasek, Jessica Robinson, Aubrey Dissinger, Lauren George, and Dr. Michal Wojno) for their support and for answering my many questions when I was navigating this experience. Thank you especially to Dr. Kwasek for her help in designing this experiment and advising me through it; our lab technician Jessica Robinson for training me, setting things up for my experiment, and helping me find whatever I needed; and Dr. Wojno for decapsulating the Artemia cysts for me to hatch them. I’d additionally like to thank the Hamel Center for Undergraduate Research and Mr. Dana Hamel for their generosity in funding my project and trusting me to do quality research. I’m grateful to my friends and family for supporting me, especially my parents for letting me drive their car and always having a meal waiting for me when I got home, my girlfriend for listening to my endless talk about my study, and my buddy Brian for putting up with me showing up to his grad party late and smelling like fish. Lastly, thank you to Dr. Jessica Bolker for personally introducing me to Dr. Kwasek and always being there for me throughout my time at UNH.
References
Bambino, K., & Chu, J. (2017). Zebrafish in Toxicology and Environmental Health. Current Topics in Developmental Biology, 124, 331–367. https://doi.org/10.1016/bs.ctdb.2016.10.007
Conceição, L. E. C., Yúfera, M., Makridis, P., Morais, S., & Dinis, M. T. (2010). Live feeds for early stages of fish rearing. Aquaculture Research, 41(5), 613–640. https://doi.org/10.1111/j.1365-2109.2009.02242.x
Houde, E. D. (1972). Some Recent Advances and Unsolved Problems in the Culture of Marine Fish Larvae1. Proceedings of the Annual Workshop - World Mariculture Society, 3(1–4), 83–112. https://doi.org/10.1111/j.1749-7345.1972.tb00050.x
Howe, K., Clark, M. D., Torroja, C. F., Torrance, J., Berthelot, C., Muffato, M., Collins, J. E., Humphray, S., McLaren, K., Matthews, L., McLaren, S., Sealy, I., Caccamo, M., Churcher, C., Scott, C., Barrett, J. C., Koch, R., Rauch, G.-J., White, S., … Stemple, D. L. (2013). The zebrafish reference genome sequence and its relationship to the human genome. Nature, 496(7446), 498–503. https://doi.org/10.1038/nature12111
J. Dhont, K. Dierckens, J. Støttrup, G. Van Stappen, M. Willie, & P. Sorgeloos. (2013). Rotifers, Artemia and copepods as live feeds for fish larvae in aquaculture. In Advances in Aquaculture Hatchery Technology (pp. 157–202). Woodhead Publishing. https://doi.org/10.1533/9780857097460.1.157
Kalueff, A. V., Stewart, A. M., & Gerlai, R. (2014). Zebrafish as an emerging model for studying complex brain disorders. Trends in Pharmacological Sciences, 35(2), 63–75. https://doi.org/10.1016/j.tips.2013.12.002
Ma, K. G. L., Lieggi, C., Lertpiriyapong, K., Afolalu, A. A., Riedel, E. R., & Lipman, N. S. (2023). Successful Rearing of Nutritionally Supplemented Rotifers (Brachionus plicatilis) at Reduced Salinity for Zebrafish (Danio rerio) Polyculture. Zebrafish, 20(6), 250–259. https://doi.org/10.1089/zeb.2023.0027
Major, R. J., & Poss, K. D. (2007). Zebrafish Heart Regeneration as a Model for Cardiac Tissue Repair. Drug Discovery Today. Disease Models, 4(4), 219–225. https://doi.org/10.1016/j.ddmod.2007.09.002
Modarresi Chahardehi, A., Arsad, H., & Lim, V. (2020). Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants, 9(10), 1345. https://doi.org/10.3390/plants9101345
Nusslein-Volhard, C., & Dahm, R. (2002). Zebrafish. OUP Oxford.
Sorgeloos, P., Bossuyt, E., Laviña, E., Baeza-Mesa, M., & Persoone, G. (1977). Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture. Aquaculture, 12(4), 311–315. https://doi.org/10.1016/0044-8486(77)90209-5
Author and Mentor Bios

Esher Swanson is pursuing a double major in sustainability and marine, estuarine, and freshwater biology. Born in Great Barrington, Massachusetts, he moved to Nebraska a month before he turned six and grew up visiting and volunteering at Omaha's Henry Doorly Zoo and Aquarium, currently the #1 zoo in the nation. There he developed an appreciation for animals, their behavior, and their habitats, as well as sustainability. Since he moved to Milton, NH, and started at UNH, he has been heavily involved on campus. Esher is currently on the executive board for the Organic Garden Club (OGC); secretary of the Honors Undergraduate Social Committee (HUSC); an intramural soccer captain; and a member of club fencing. He is a former member of the Zoological Society, Perry the Platypus Society, and intramural volleyball team. In addition to his URA study with Dr. Kwasek, Esher is part of Dr. Nathan Furey’s Fish and Movement Ecology Lab, a proud Shoals Marine Lab alum of the last three years, and part of the Aquaculture and Engaged Research Honors Cocurricular Experience.
Karolina Kwasek completed her undergraduate and graduate studies in inland fisheries at University of Warmia and Mazury in Olsztyn, Poland, followed by a PhD in animal science at The Ohio State University. After several years working in the private sector and for non-profit organizations involved with aquaculture, she returned to academia, first at Southern Illinois University and currently as assistant professor at the University of New Hampshire, where she established an externally funded research program in aquaculture. Her lab has received many resources from state and federal agencies, as well as from industry (fish, feed, and raw material producers), to tackle aquaculture challenges on a local, national, and global level. She has worked with multiple species (trout, salmon, yellow perch, walleye, largemouth bass, tilapia, zebrafish, and shrimp) using both lab-based as well as hatchery-like research culture systems, with emphasis on improving hatchery technology.
Copyright © 2025, Esher Swanson