Batteries, those ubiquitous add-ons to virtually every electronic birthday or holiday gift, have been getting a bad rap of late. Recent headlines have detailed their propensity to explode, wreaking havoc in cell phones, hover boards and automobiles. And then there’s the environmental-unfriendliness factor: Most common batteries contain toxic metals that are harmful if not disposed of properly.

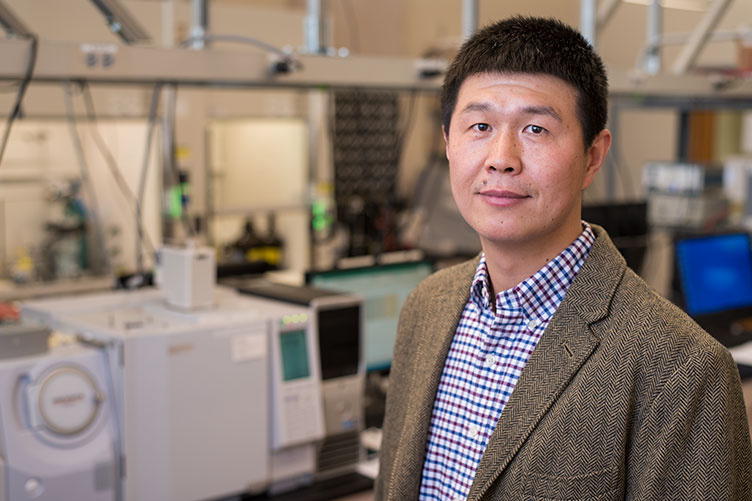
At UNH, a trio of researchers has created an alternative energy storage system that could lead to battery technology superior to at least some segment of what is currently on the market. Led by Xiaowei Teng, associate professor of chemical engineering, the team set out to develop a solution for a rechargeable energy storage device that would offer enhanced safety yet be reliable and low cost by modifying a unique form of manganese oxide known as Mn5O8.
“This manganese oxide mineral was first studied back in 1965, but since then very few people have considered it in designing today’s rechargeable energy storage,” Teng says. “The challenge with creating electrode materials for water-based energy storage like this one is being able to get enough charge, or discharge, cycles and a good amount of storage capability. So we thought altering the way the Mn5O8 was prepared for use in battery electrodes might make it a viable option.”

Most batteries work by placing two different metals—the electrodes—into a substance—the electrolyte—to create chemical reactions that release energy. Aqueous electrochemical energy storage devices have attracted attention lately because their electrolyte is water-based and therefore less likely to combust once exposed to air or moisture. Rechargeable aqueous batteries, especially ones using Earth-abundant and nontoxic materials, have shown great promise owing to their high safety, low cost and environmental friendliness. Besides having more potential to cause a fire, lithium batteries are made from an expensive element that is in scarce supply. Cost-effective energy storage has long been described as the key for the widespread adoption of renewable energy practices.

Teng worked with Xiaoqiang Shan and Daniel Scott Charles, both doctoral candidates in the chemical engineering department, to modify manganese oxide to make it less reactive in water and therefore less prone to energy-sapping decomposition. Their results, recently published in Nature Communications, yielded the blueprint for a more reliable aqueous battery with greater energy storage and increased power performance and charge cycles.
While UNH has patented this technology, don’t expect to find the team’s batteries in your local Radio Shack just yet. Teng says the application for his team’s findings is limited, because water has unique weakness as an electrolyte, and Mn508 batteries will likely never supplant their lithium-ion counterparts. “But as the industrial world becomes increasingly electrified, we will need energy storage systems with a variety of characteristics,” he says.
Originally published in UNH Magazine Spring 2017 Issue
-
Written By:
Robbin Ray ’82 | UNH Marketing | robbin.ray@unh.edu | 603-862-4864